Can Intermittent Fasting Stave Off Alzheimer’s Disease?

A study finds that intermittent fasting may be the secret to preventing Alzheimer’s disease. Photo: Tetiana Kreminska/Getty Images
Have you ever wondered why some people get Alzheimer’s disease (AD), while others are spared from it? With someone developing the disease every 65 seconds on average – which translates to about 6.2 million people aged 65 or older in the United States – it’s an important question. Alzheimer’s disease is something that is likely to affect all our lives in one way or another at some point.
For people with early-onset familial Alzheimer’s disease, which represents about 1-5% of all Alzheimer’s cases, there is a genetic component. Mutations within three specific genes can determine definitively whether you will have AD. However, for the other 95-99%, scientists don’t yet fully understand what causes it, but suspect that it is caused by a combination of many things, such as age, family history, inflammation in the brain, cardiovascular disease, traumatic brain injury, and lifestyle factors such as smoking, poor diet, lack of physical activity, high alcohol consumption, etc.
Now, the important question is: if the cause of AD is related to lifestyle choices, are there risk factors for AD that a person can control to reduce their chances of developing AD?
As it turns out, scientists say there is. While there is no cure for AD, there are ways to reduce your risk for the condition – one of which is intermittent fasting (IF). In a recent ground-breaking study, published in Cell, researchers discovered that when you eat, and how often you eat, could make a difference in your risk for AD.
The Alzheimer’s Disease Amyloid Hypothesis
When it comes to diseases, Alzheimer’s disease is a bleak one. It is an incurable degenerative disease that is common among older adults, typically 65 years and older. The reason AD is thought to be related to age is because your brain, like a car, a cell phone, or any other tool that you use often, is likely to break down the longer you have and use it. Parts wear out, screens crack, and batteries die. The same thing happens to your brain – it accumulates damage over the course of a lifetime and around the age of 65, signs of aging and deterioration begin to appear.
One prevailing theory for the cause of AD is the amyloid hypothesis, which postulates that the buildup of the peptide amyloid-beta in the brain is the main cause of the condition. Scientists have known that in patients with AD, glucose metabolism slows down, making it harder for their brain cells to generate energy. This slowed glucose metabolism has been shown to be linked to a build-up of amyloid-beta protein between nerve cells (called plaque), as well as the build-up of hyperphosphorylated tau protein inside nerve cells (called tangles) – both of which are key biomarkers of AD. Scientists have long thought that the accumulation of these plaques and tangles wreak havoc on the brain, killing cells and disrupting normal brain function as they spread.
Another chemical in the brain, acetylcholine, is also thought to be a key player in AD. High concentrations of acetylcholine is important for learning and memory. However, in people with AD, nerve cells that make acetylcholine are lost – typically due to age-related factors – and because of this, people with AD have low levels of acetylcholine.
Alzheimer’s Is Treatable, But There’s No Cure
Despite having an idea of what happens in the brains of people with AD, there aren’t very many treatment options for the 6.2 million people who live with the disease – which causes ever-worsening memory loss, cognitive and behavioral problems, confusion, difficulty with language, problems with reading, writing, and communication, and an inability to concentrate and perform daily activities. There are a handful of commonly used drugs that can ease AD symptoms such as:
- Donepezil (Aricept™), a cholinesterase inhibitor used to delay the progression of memory loss and loss of other cognitive functions by preventing an enzyme, called acetylcholinesterase, from breaking down acetylcholine.
- Rivastigmine (Exelon™), also a cholinesterase inhibitor that is used to improve memory, awareness, and the ability to perform daily activities.
- Aducanumab (Aduhelm™), a monoclonal antibody that slows down the decline of cognitive skills and functional abilities. It works by sticking to, and removing, amyloid-beta proteins from the brain to prevent build up and plaque formation.
Unfortunately, however, none of these – or other therapies for that matter – have consistently shown to be successful at slowing down the disease’s progression. Meaning that these drugs may work in a small number of patients with AD for a short period of time, but in a larger portion of patients, they are ineffective.
Out With the Old Amyloid Hypothesis and in With the New Intermittent Fasting Theory
With the increasing number of AD treatments continuing to fail, scientists began to broaden their list of the condition’s potential causes, such as whether glucose metabolism could be the key to understanding AD. This led scientists to studying intermittent fasting (IF) – an eating pattern of switching between periods of eating and fasting on a regular schedule – and its effect on metabolism, and on the brain.
The new hypothesis goes like this: the most important known risk factor for AD is aging. In fact, the number of people with AD doubles about every 5 years beyond the age of 65, with about one-third of all people aged 85 and older having the condition. Aging happens in part because the buildup of damaged bits of biological material outpaces the body’s ability to break them down and remove them. This buildup is all but non-existent in children, slow in young to middled-aged adults, but is kicked into to high gear in old age because the body’s ability to remove the damaged bits and pieces declines with age.
Since many studies in aging research have found that turning on a natural process called autophagy can help the body rid itself of old, dying, damaged cells and biological materials, it stands to reason that “turning on” autophagy could possibly reduce your risk of AD.
One way to “flip on the autophagy switch” is through intermittent fasting (IF).
How Does Fasting Affect the Body and the Brain?
There are many approaches to intermittent fasting, such as eating during only certain hours of the day, refraining completely on certain days, or drastically reducing calories on a few days of the week. The idea is that abstaining from food for longer-than-normal periods of time changes the body’s metabolism. When you stop eating, your body can no longer rely on carbohydrates and sugar for energy. Instead, your body – brain included – begins relying on ketone bodies for energy, which are derived from stored fat.
When the brain shifts to ketone metabolism, brain cells enter a low-energy, protective state. So, instead of using a lot of energy to create new proteins for the brain to use, these brain cells enter an energy-conserving mode and use the worn-out, damaged bits of protein. In doing so, the old, damaged bits and pieces of cell proteins within the brain are cleaned up and cleared out – this process of ketone metabolism describes how the brain undergoes autophagy.
And so, scientists hypothesized that intermittent fasting could effectively shift your brain cells into this protective, autophagous state, which would help prevent or slow cognitive decline by keeping brain cells clear of damaged junk proteins, such as amyloid-beta, for longer.
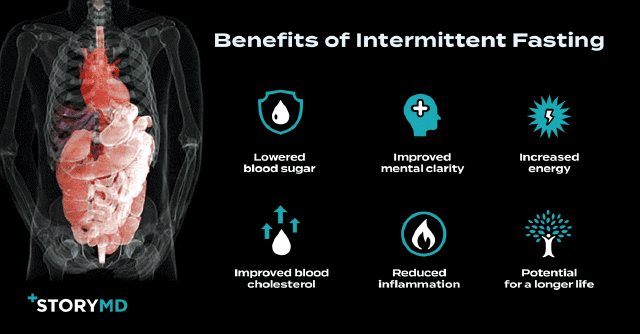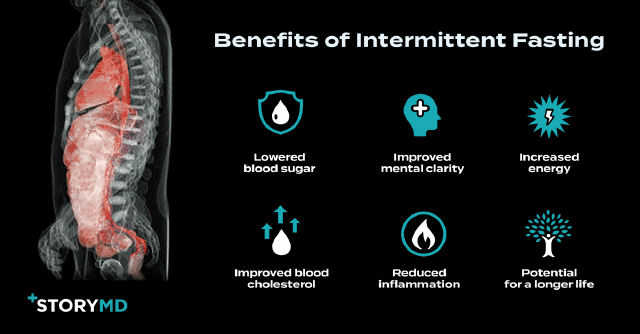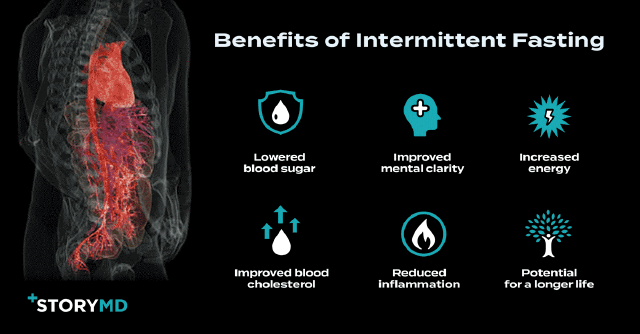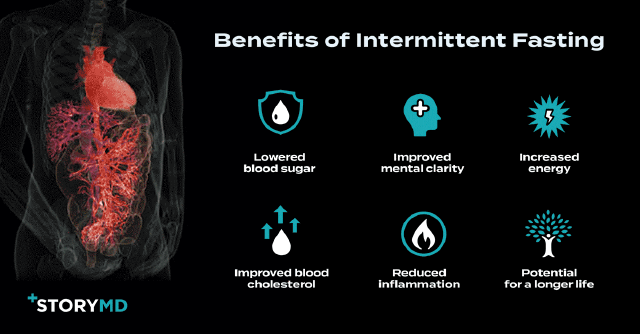
Study Finds Intermittent Fasting May Be the Secret to Preventing Alzheimer’s Disease
To test this hypothesis, a team of researchers, led by Dr. Valter Longo in collaboration with Drs. Christian Pike and Pinchas Cohen from the University of Southern California Leonard Davis School of Gerontology, conducted a study to determine whether intermittent fasting could, in fact, remove the buildup of the two key hallmarks of AD – amyloid-beta and hyperphosphorylated tau proteins.
The researchers used three groups of mice, one group were healthy mice fed a standard diet, and the other two groups of mice were genetically engineered to develop Alzheimer’s and were fed a fasting-mimicking diet (FMD) for four to five days at a time, twice a month, and between these cycles, they ate a regular diet. The FMD was high in unsaturated fats and low in overall calories, proteins, and carbohydrates, which mimics the impact of sticking to a fasting diet while still providing the mice with their necessary nutrients.
Intermittent Fasting Reduces Two Key Biomarkers of AD
The results showed that both groups of Alzheimer’s mice, while on the FMD, displayed a significant decrease in both amyloid-beta and hyperphosphorylated tau proteins. The researchers also found that the mice on the fasting diet had lower levels of brain inflammation.
The FMD mice even had lower levels of oxidative stress, which the researchers say plays a role in the onset of Alzheimer’s. Oxidative stress, which develops due to an imbalance between the production and accumulation of oxygen reactive species (ROS), damages neurons and leads to more amyloid-beta building up in the brain.
Additionally, when studying the behaviour of the mice, the researchers found that the fasting mice displayed less cognitive decline than mice on the standard diet. The fasting mice performed better during a maze test in comparison to Alzheimer’s mice on a standard diet and nearly matched the performance of healthy mice.
Dr. Longo and colleagues concluded that the FMD cycles appeared to be an effective means of reversing two key biomarkers of AD and cognitive function in two of the major mouse models for AD. While the results are very promising, mice are not humans. The researchers now hope to recreate this study with human participants to further explore the effects of IF on preventing AD.
Zoomer.Health provides vetted scientific medical information across nearly 5,000 health and wellness topics. To read more, click here.
RELATED:
Alzheimer’s Awareness Month: How Much Memory Loss Is Normal With Aging?
The Skinny on Intermittent Fasting: How the “When of Eating” Can Impact Your Weight
A New Canadian Program Helps People Living With Alzheimer’s Disease